Help patients gain control of their day.
Treat their anxiety with LOREEV XR®, a once-daily extended-release capsule.1


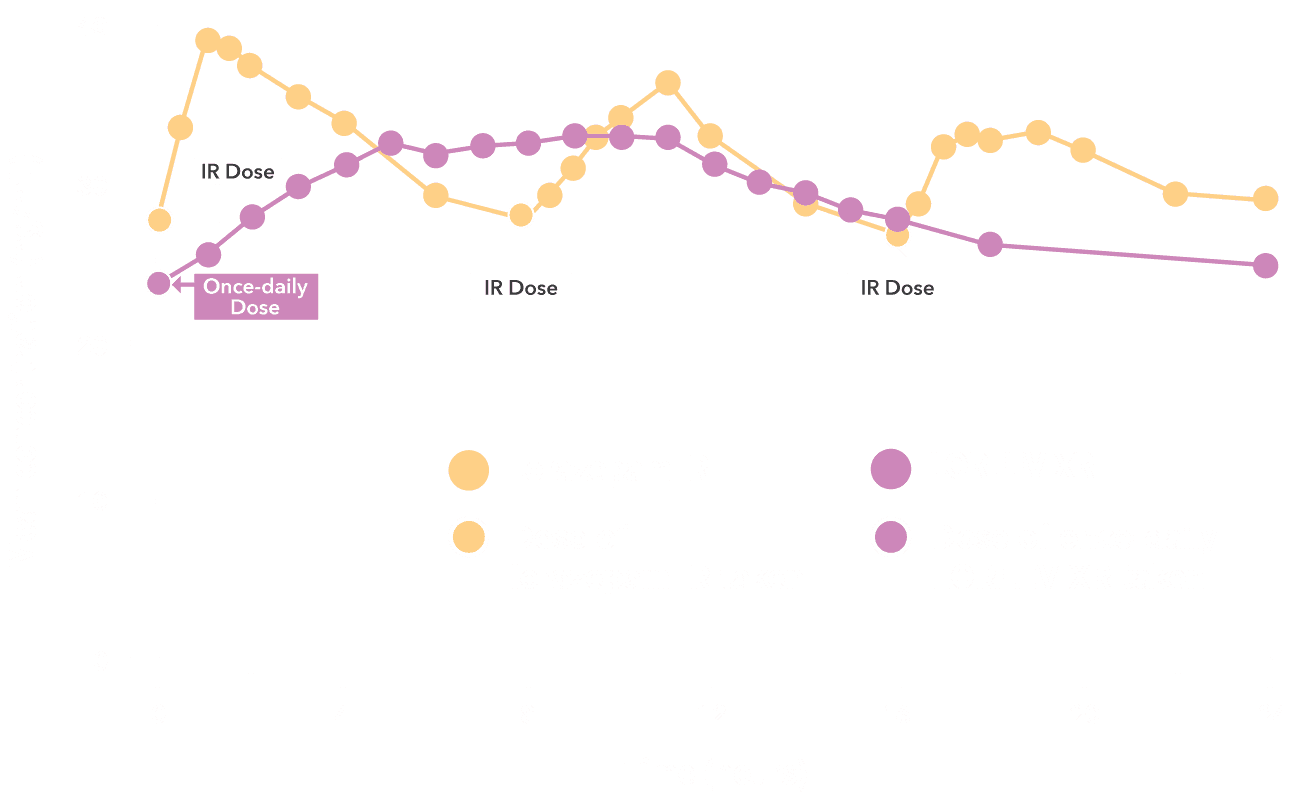
Two-bead extended-release delivery system
LOREEV XR combines 2 types of extended-release beads to provide a smooth 24-hour profile2
Metabolism
Approximately 70% to 80% of drugs in clinical use are metabolized through the cytochrome P450 (CYP450) pathway.5 Lorazepam is not.13
Why does this matter?

Lorazepam is metabolized differently from these common benzodiazepines, SSRIs, and SNRIs.
| Primary Metabolic Pathways | ||||
| Medication | CYP450 2C19 | CYP450 2D6 | CYP450 3A4 | Glucuronidation via UGTs |
| Benzodiazepines | ||||
| Lorazepam1 | ||||
|---|---|---|---|---|
| Alprazolam9 | ||||
| Clonazepam10 | ||||
| Diazepam11 | ||||
| SSRIs and SNRIs | ||||
| Citalopram12 | ||||
| Duloxetine13 | ||||
| Escitalopram14 | ||||
| Fluoxetine15 | ||||
| Fluvoxamine16 | ||||
| Levomilnacipran17 | ||||
| Paroxetine18 | ||||
| Sertraline19 | ||||
| Vilazodone20 | ||||
| Vortioxetine21 | ||||
| Venlafaxine22 | ||||
| SSRIs=selective serotonin reuptake inhibitors; SNRIs=serotonin-norepinephrine reuptake inhibitors; UGTs=UDP-glucuronosyltransferases. | ||||
Help your patients manage their medication costs

With the LOREEV XR Patient Savings Program, eligible insured patients pay as little as $20/month for LOREEV XR*
* Applies to commercially insured patients. Individual costs may vary. Program eligibility and restrictions apply.
Important Safety Information
Indication for Use
LOREEV XR is indicated for the treatment of anxiety disorders in adults who are receiving stable, evenly divided, three times daily dosing with lorazepam tablets.
Contraindications
LOREEV XR is contraindicated in patients with:
- Hypersensitivity to benzodiazepines or any ingredients in LOREEV XR
- Acute narrow-angle glaucoma
Warnings and Precautions
Central Nervous System (CNS) Depression
- LOREEV XR may produce CNS depression. Caution against engaging in hazardous occupations or activities requiring complete mental alertness.
- Use alone and with other CNS depressants may lead to potentially fatal respiratory depression. Alcohol should be avoided, and other CNS depressants used with caution.
Patients with Depression or Psychosis
- LOREEV XR is not recommended in patients with a primary depressive disorder or psychosis. Preexisting depression may emerge or worsen.
- A possibility for suicide should be kept in mind in patients with depression. Benzodiazepines should not be used without adequate antidepressant therapy.
Risk of Paradoxical Reactions
- Paradoxical reactions have occasionally been reported during benzodiazepine use and are more likely to occur in the elderly. If this occurs, discontinue LOREEV XR.
Allergic Reactions to FD&C Yellow No. 5 (Tartrazine)
- LOREEV XR 1 mg capsules contain FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions in certain individuals and is seen frequently in patients who also have aspirin hypersensitivity.
Neonatal Sedation and Withdrawal Syndrome
- LOREEV XR use during later stages of pregnancy can result in sedation and/or withdrawal symptoms in the neonate. Monitor neonates during pregnancy and labor for signs of sedation and withdrawal.
Risk in Patients With Impaired Respiratory Function
- Closely monitor patients taking LOREEV XR for impaired respiratory function, and consider discontinuing it if signs and symptoms of respiratory depression or apnea occur.
Laboratory Tests
- Leukopenia and elevations of lactase dehydrogenase (LDH) have developed in patients receiving lorazepam tablets. Periodic blood counts and liver function tests are recommended during long-term therapy.
Adverse reactions
Most frequent adverse reactions in clinical trials were sedation (15.9%), dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%).
Drug interactions
Avoid initiation of UDP-glucuronosyltransferase (UGT) inhibitors. Dose reduction requires switching to lorazepam tablets for dose adjustment.
Use in specific populations
Because of the potential for serious adverse reactions, breastfeeding is not recommended during treatment with LOREEV XR.
For additional safety information about LOREEV XR, see the LOREEV XR Full Prescribing Information including Boxed Warning and Medication Guide.
You are encouraged to report negative side effects of prescription drugs to Almatica at 1-877-447-7979, or the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.